Master Data Management
Master Data Management ensures structured and standardized handling of laboratory master data. It includes Products, Specifications, and Equipment, each serving a distinct function within the system.
Products
The Products module serves as a centralized database of all registered products. This ensures that every product used in laboratory processes is documented, categorized, and easily accessible.
Users can:
- Register and manage product names, dosage forms, and packaging types.
- Ensure traceability of products across orders, specifications, and test results.
Below is an example of the Products module interface:
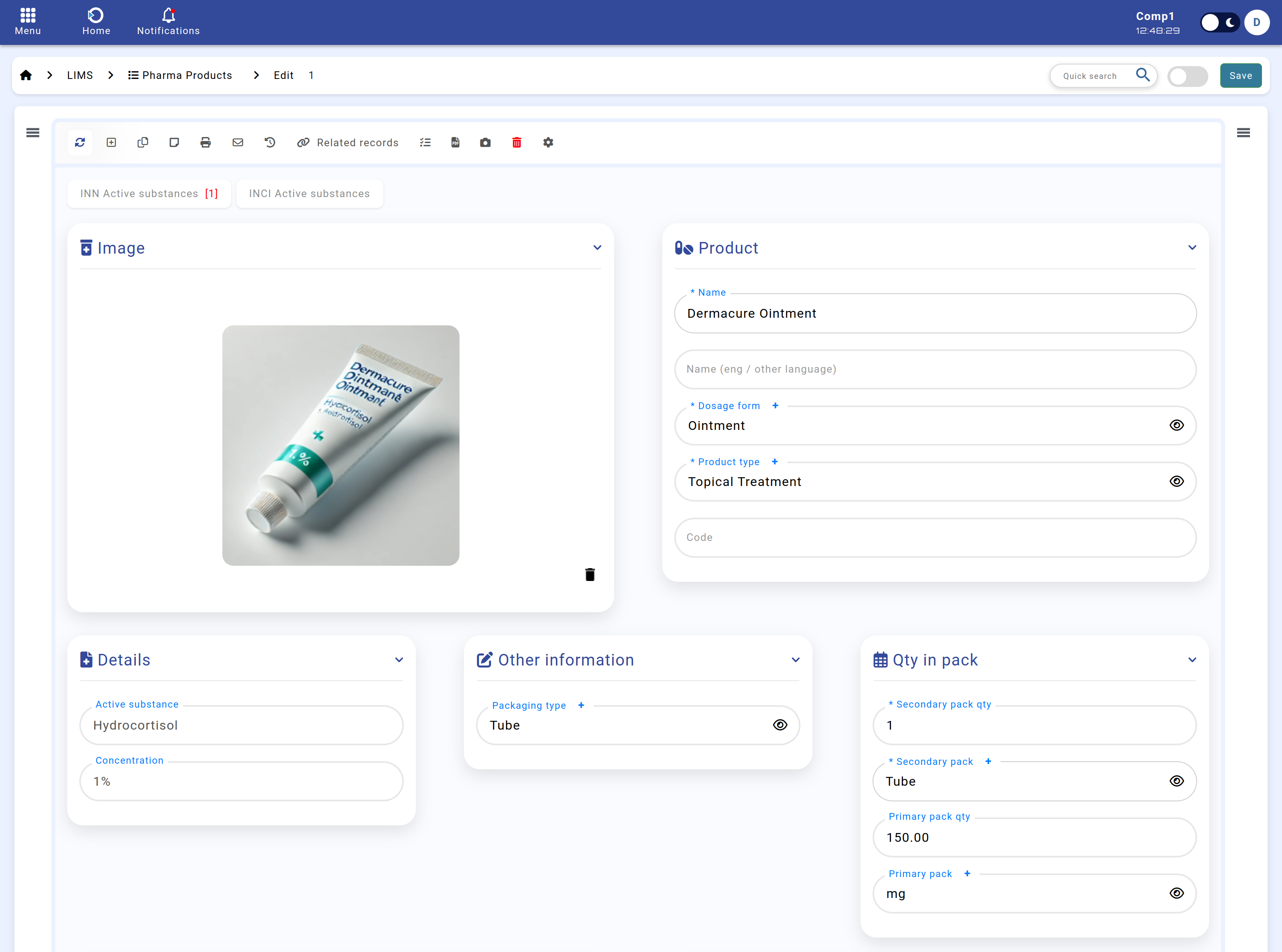
Figure: Products module interface.
Specifications
The Specifications module defines testing requirements and parameters for each product. Specifications serve as predefined templates for laboratory orders, ensuring all tests are conducted consistently.
Users can:
- Define testing specifications for products, including required tests and acceptance criteria.
- Set up parameters for chemical or microbiological analysis.
- Automatically link specifications when generating new laboratory orders.
Below is an example of the Specifications module interface:
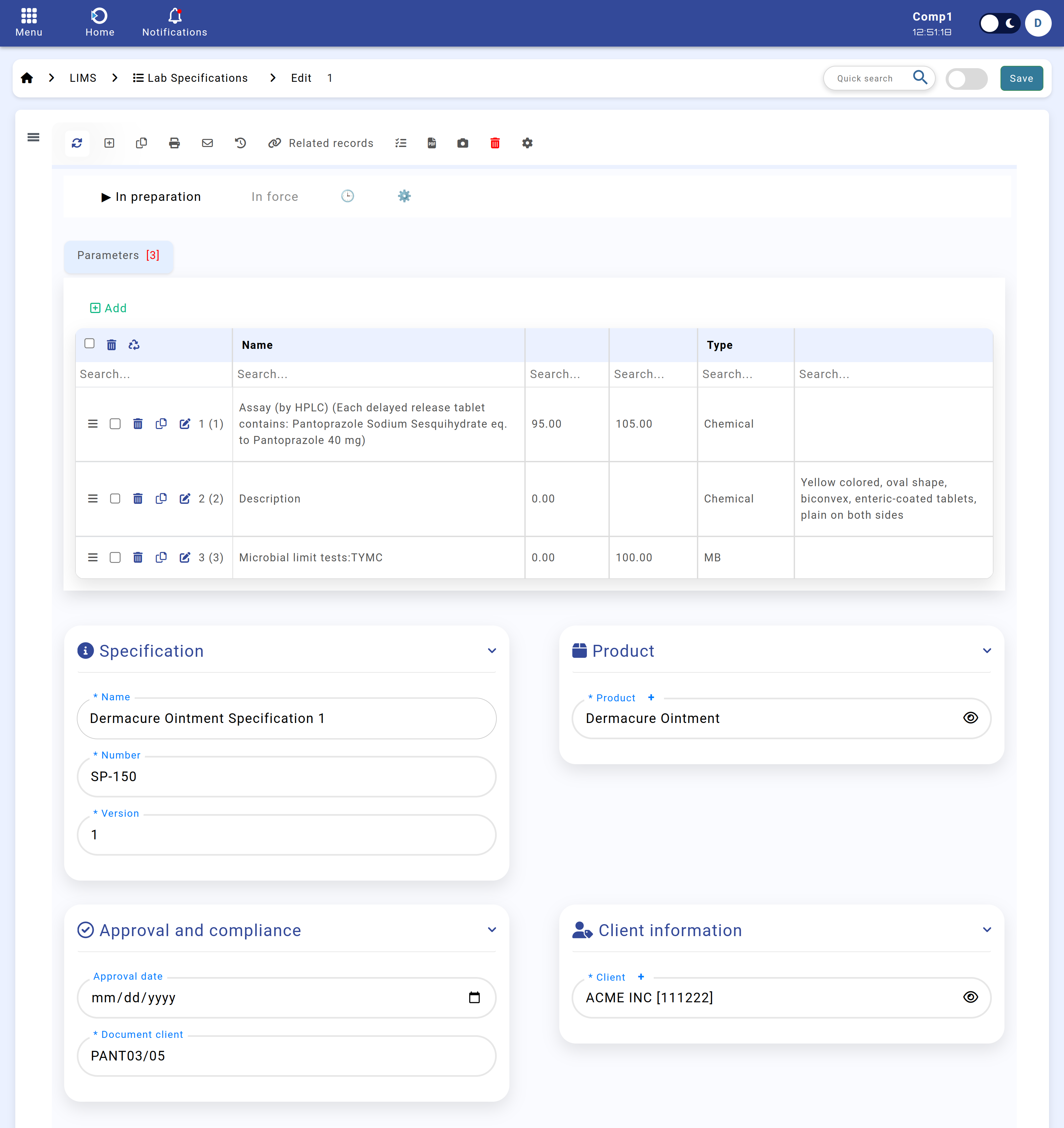
Figure: Specifications module interface.
Equipment
The Equipment module is used to track laboratory instruments, software validation, calibration schedules, and maintenance activities. Proper equipment tracking ensures compliance, reduces downtime, and maintains testing accuracy.
Users can:
- Register laboratory equipment, including manufacturer details, model numbers, and serial numbers.
- Monitor qualification and calibration schedules.
- Track software validation to maintain regulatory compliance.
Below is an example of the Equipment module interface:
Figure: Equipment module interface.
