Batch record management
/Audit Trail & Compliance
Audit Trail & Compliance
ProFlow Batch Management ensures robust compliance with regulatory standards by maintaining a comprehensive and immutable audit trail. Every action performed within the system is recorded, offering full traceability to meet the most stringent audit requirements.
Ensuring Regulatory Compliance
ProFlow Batch Management is designed to meet global regulatory standards, including:
- EU GMP & Annex 11: Adheres to European guidelines for Good Manufacturing Practices, ensuring secure electronic records.
- FDA 21 CFR Part 11: Complies with U.S. requirements for electronic records and signatures, guaranteeing data integrity and accountability.
These compliance measures ensure that companies can confidently meet audit expectations while maintaining operational efficiency.
Audit Trail Features
The audit trail in ProFlow captures every action performed in the system, ensuring complete traceability with the following key features:
- Full Activity Tracking: Records the who, what, and when for every action, providing a detailed history of user interactions.
- Immutable Logs: All logs are tamper-proof, ensuring the integrity and reliability of audit data.
- Automatic Record Keeping: Includes approvals, rejections, and other critical actions, enabling a transparent workflow.
With these features, ProFlow ensures companies can meet regulatory requirements and internal quality standards effortlessly.
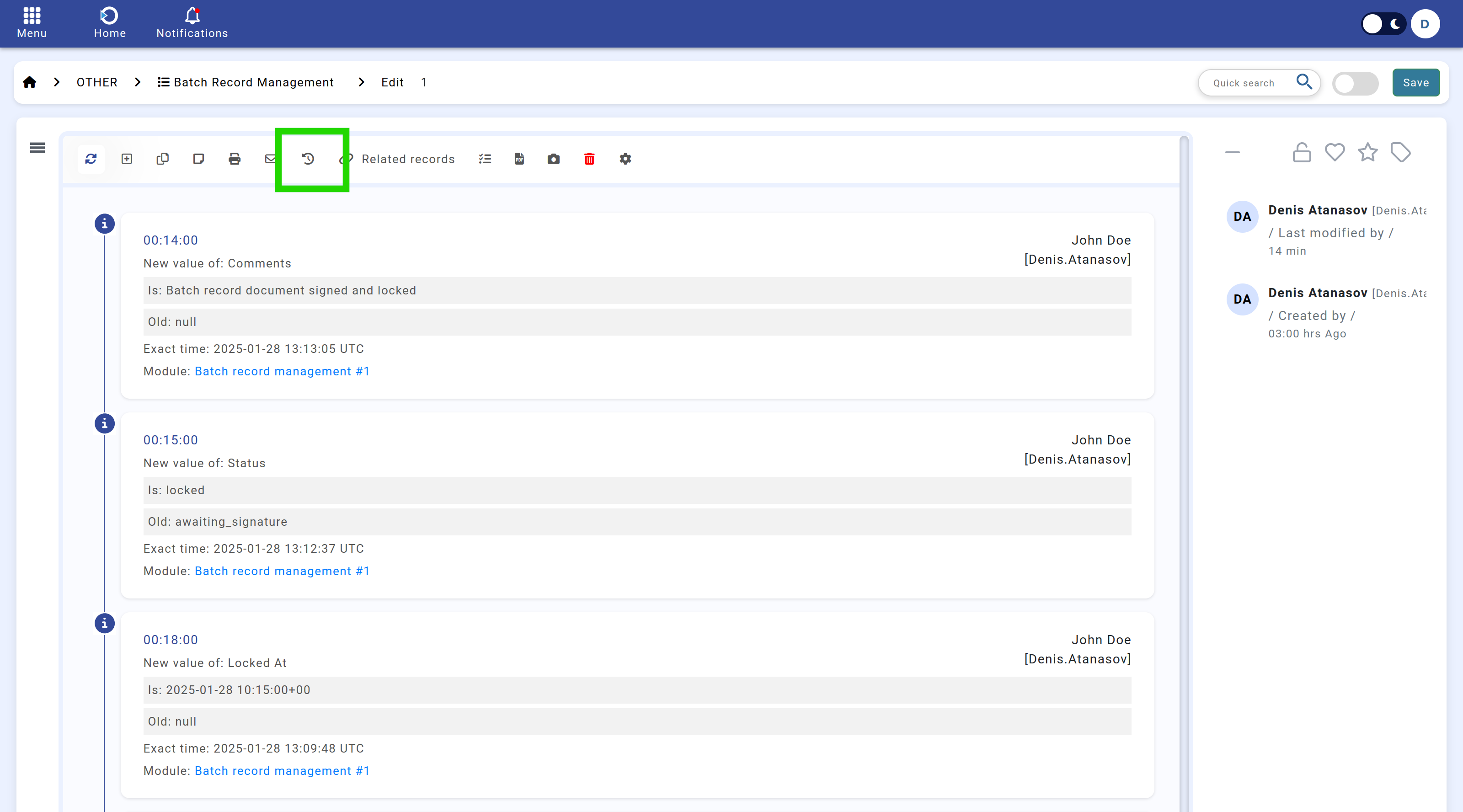
Navigation: Home screen > Batch Record Management > Activity.
Why It Matters
The detailed audit trail maintained by ProFlow not only ensures compliance but also provides operational insights by enabling companies to:
- Quickly identify the source of errors or issues during audits or inspections.
- Demonstrate accountability through a detailed log of all user actions.
- Reduce the risk of compliance violations by maintaining transparent records.
