Batch record management
/Batch Record Lifecycle
Batch Record Lifecycle
The batch record lifecycle follows a structured process to ensure full traceability, compliance, and efficiency in pharmaceutical manufacturing. Each stage enforces quality control measures, ensuring records are securely documented, reviewed, approved, and locked in accordance with regulatory standards.
Process Flow Overview
1. Batch Record Creation
When a manufacturing process is completed, the system automatically generates a batch record, capturing key data such as:
- Equipment Type: The machine used during production.
- Document Type: The specific type of batch-related document (e.g., Cleaning Report, Calibration Record).
- Batch Number: A unique identifier for tracking production runs.
- Timestamp: The exact date and time of record generation.
2. Approval Assignment (Automated Mapping)
The system streamlines the approval process by automatically assigning the correct approver based on:
- Equipment Type: Ensures that only qualified personnel review equipment-specific records.
- Batch Document Type: Approvers are dynamically selected based on the document’s nature and compliance requirements.
3. Approval Workflow & Review
Once assigned, the designated approvers receive automated notifications prompting them to review the batch documentation. Approvers have the option to:
- Approve: If the record meets all requirements, the process moves forward.
- Reject: If compliance requirements are not met, the batch record is rejected, and corrective actions are required.
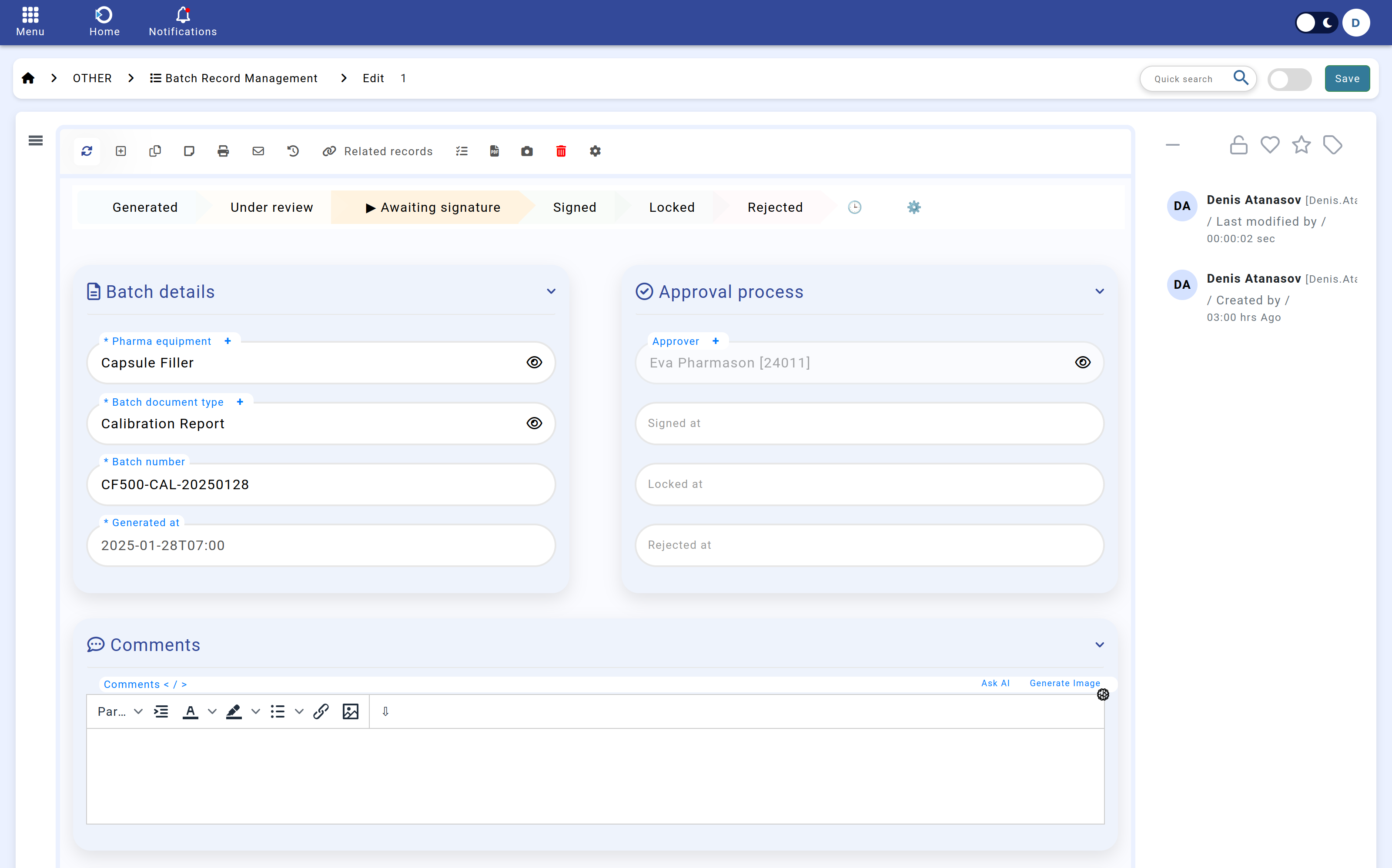
Navigation: Home screen > Batch Record > Edit.
4. Digital Signing Process
Once the record is approved, it progresses to the digital signing stage, ensuring full traceability and regulatory compliance. The system:
- Generates a tamper-proof signature request for authorized users.
- Supports Qualified Electronic Signatures (QES) to meet global regulatory standards.
- Ensures all signatures are time-stamped and logged for audit purposes.
5. Final Locking & Archival
Once signed, the batch record enters its finalized state, preventing any further modifications. The system:
- Locks the record: Ensuring data integrity and preventing unauthorized changes.
- Maintains full traceability: Storing a compliance audit trail for all approvals, modifications, and sign-offs.
- Provides controlled access: Users can retrieve records for review while maintaining compliance safeguards.
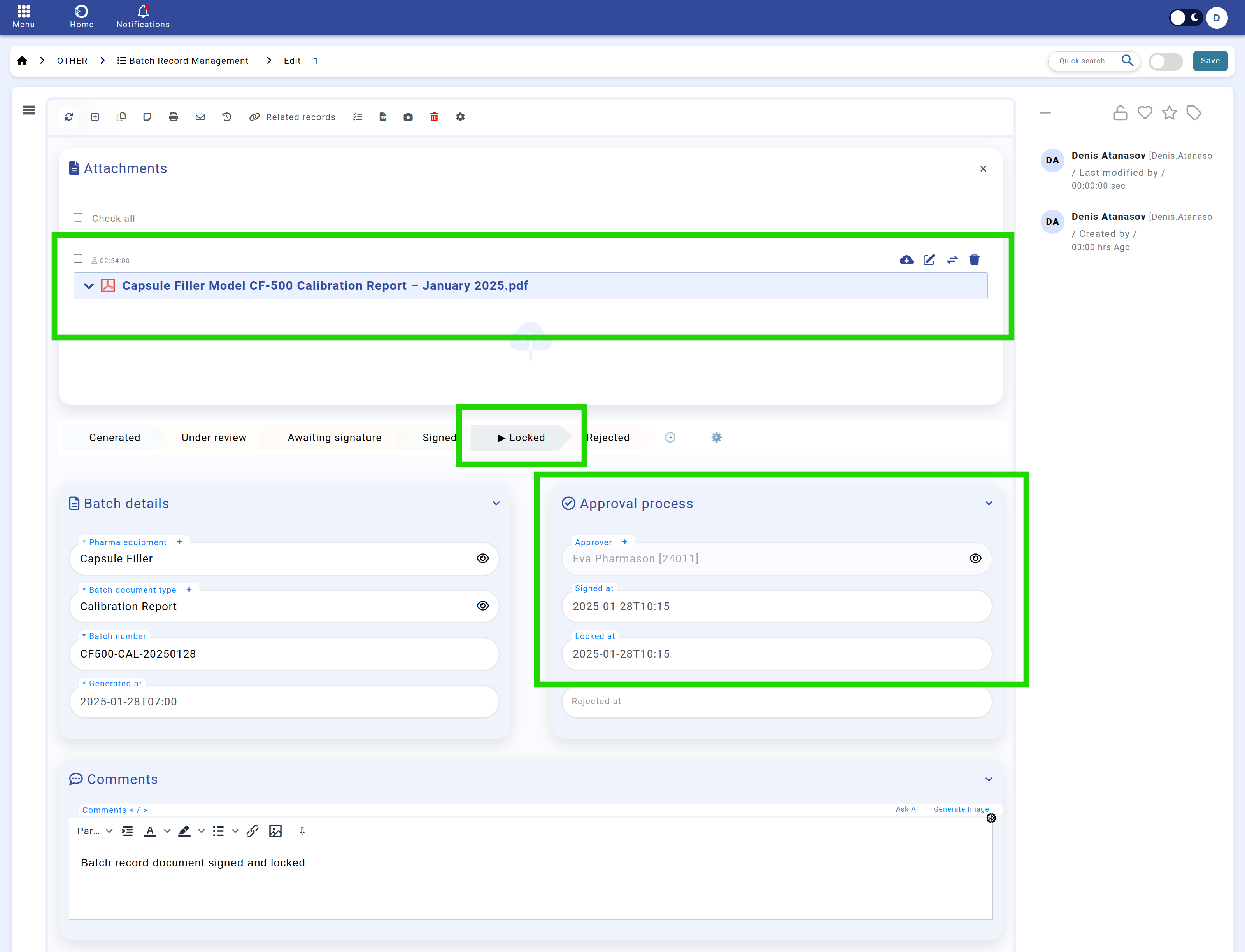
Navigation: Home screen > Batch Record > Locked & Archived.
