ProFlow Document Management
/Audit Trail
Audit Trail
This section provides an overview of all audit-related features in the system. Below you will find details about specific audit logs or reports that track user actions and changes, ensuring compliance and traceability—particularly important in regulated environments such as pharmaceuticals.
Document Management Audit Log
The Document Management Audit Log captures a chronological record of all changes performed in the Document Management module. It details who changed what, when, and how, providing essential transparency for regulatory compliance (e.g., FDA 21 CFR Part 11) and internal quality audits.
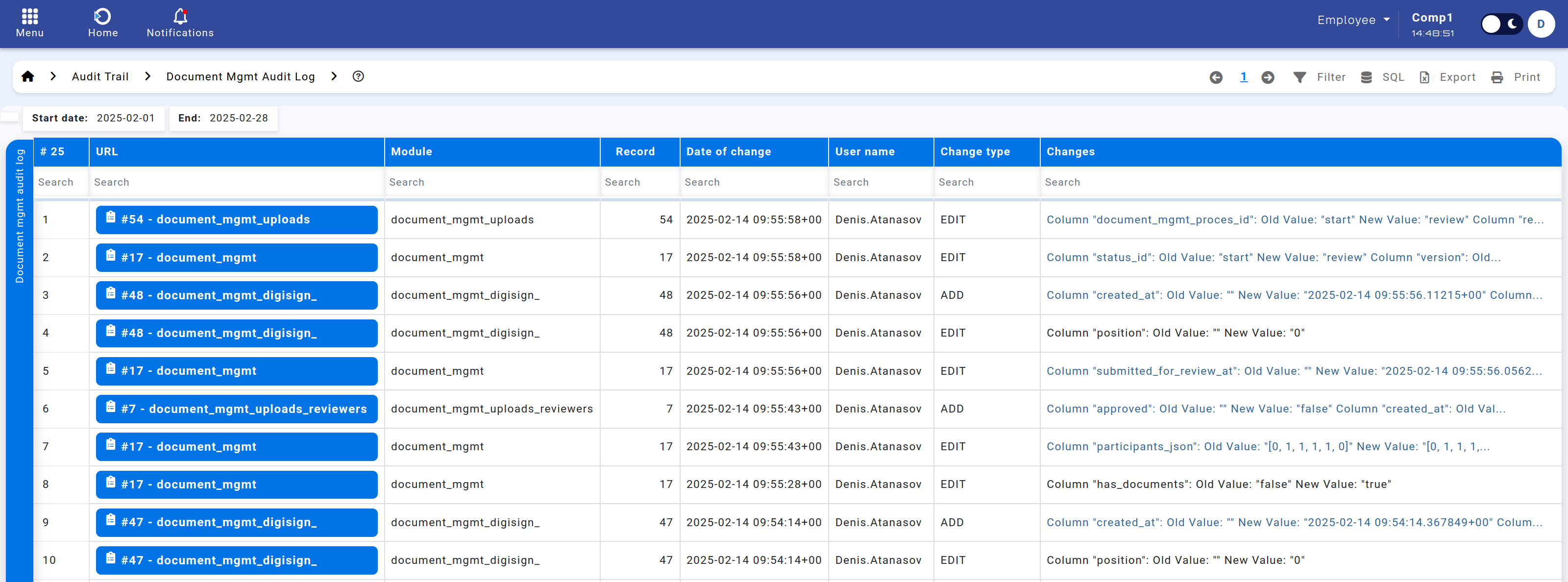
Figure: Document Management Audit Log.
Access and Navigation
- Location: From the system’s home screen, go to Audit Trail > Document Mgmt Audit Trail.
- Default View: Shows activities for the current month, sorted so the newest changes appear first.
- Filters:
- User – Narrow results to a specific user’s actions.
- Time Range – Specify start/end dates for a chosen period.
Columns in the Log
The report typically includes:
- Link – Direct reference to the record that was created, modified, or removed.
- Module – Indicates which table (e.g. document_mgmt_uploads) was affected.
- Record ID – The numeric identifier of the record within that module.
- Date of Change – The exact timestamp of the modification.
- Username – The user who performed the action.
- Change Type – Whether the action was Add, Edit, or Delete.
- Changes – Lists which fields were altered in one transaction (e.g., multiple fields updated in a single save).
Purpose and Benefits
- Transparency: View the full history of document changes and associated details.
- Traceability: Clearly see which user performed each edit, with timestamps.
- Regulatory Support: Ensures compliance with stringent industry regulations by maintaining an accurate audit log.
The Document Management Audit Log is a key component in maintaining data integrity and meeting audit requirements in pharmaceutical settings or any environment with strict documentation standards.
